Recently, Professor Tong-Bu Lu's team from the Institute for New Energy Materials and Low Carbon Technologies at our university published their latest research progress on hydrogen production via water electrolysis under neutral pH conditions in top journal JACS.

Compared to other hydrogen production methods, water electrolysis driven by renewable energy offers a green pathway for clean hydrogen production with zero carbon emissions. Among various electrolysis conditions, hydrogen evolution reaction (HER) at neutral pH has attracted significant research interest due to its ability to broaden catalyst selection, reduce corrosion of catalysts and electrolysis devices, and enable direct seawater electrolysis. Platinum-based catalysts typically exhibit excellent HER activity, but their high cost hinders large-scale application. Metallic nickel, owing to its low cost and high conductivity, is frequently used in alkaline/neutral HER catalysts. However, its HER activity remains unsatisfactory primarily due to its poor water dissociation capability. A common strategy to enhance the HER activity of metallic nickel involves introducing metal oxides to adsorb *OH, thereby promoting the water dissociation process. Nevertheless, excessively strong *OH adsorption can lead to difficult *OH desorption, consequently causing catalyst poisoning. Therefore, how these metal oxides influence the HER activity of nickel and the rationale for selecting appropriate metal oxides remain insufficiently studied.
To address it, the team led by Prof. Tong-Bu Lu and Zi-You Yu first constructed a series of Ni/MOx heterostructures (M = Ti, Cr, Mn, Ni, Zn, Cd, or In) as model catalysts to investigate the influence of different metal oxides on the HER activity of nickel. The results revealed a volcano-type relationship between the HER activity of Ni/MOx catalysts and their Lewis acidity, suggesting that Lewis acidity can serve as an effective descriptor for HER activity under neutral conditions. Among them, Ni/ZnO with the moderate Lewis acidity yields optimal *OH binding, which balances water dissociation and *OH desorption kinetics for the best HER activity, following the Sabatier principle. Subsequently, Ni/ZnO was grown in situ on a conductive nickel foam substrate to fully expose active sites. The resulting catalyst required overpotentials of only 34 and 194 mV to achieve current densities of 10 and 200 mA cm⁻2, respectively, in a neutral electrolyte, significantly outperforming the Pt/C catalyst. Furthermore, it demonstrated stable operation for 2000 h at both 10 and 200 mA cm⁻2, ranking it among the best neutral HER catalysts reported to date.
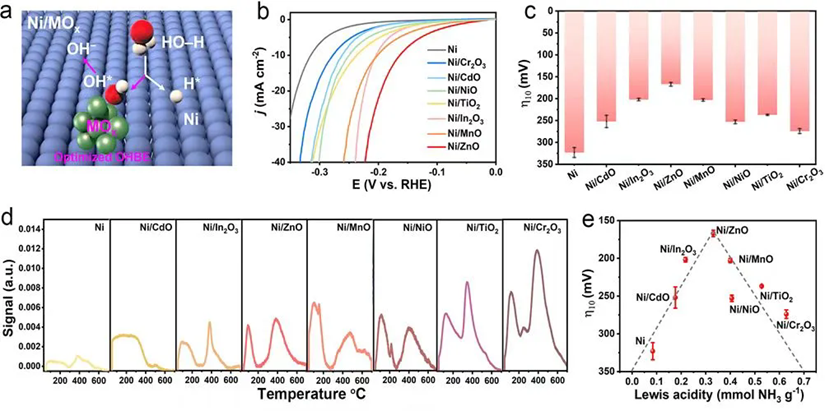
This study employed a general method to synthesize a series of Ni/MOx heterostructure catalysts and systematically investigated their HER activities in a neutral electrolyte. Further measurement of the catalysts' Lewis acidity by ammonia temperature-programmed desorption (NH3-TPD) revealed a volcano-type correlation between the Lewis acidity of the Ni/MOx catalysts and their HER activity. Ni/ZnO, located at the peak of the volcano plot, exhibited the best HER activity due to its moderate *OH binding capability, which effectively balanced the water splitting and *OH desorption processes. This finding indicates that Lewis acidity can serve as an effective descriptor for neutral HER activity, adhering to the Sabatier principle, which posits that the interaction between the catalyst and the adsorbate should be neither too strong nor too weak.
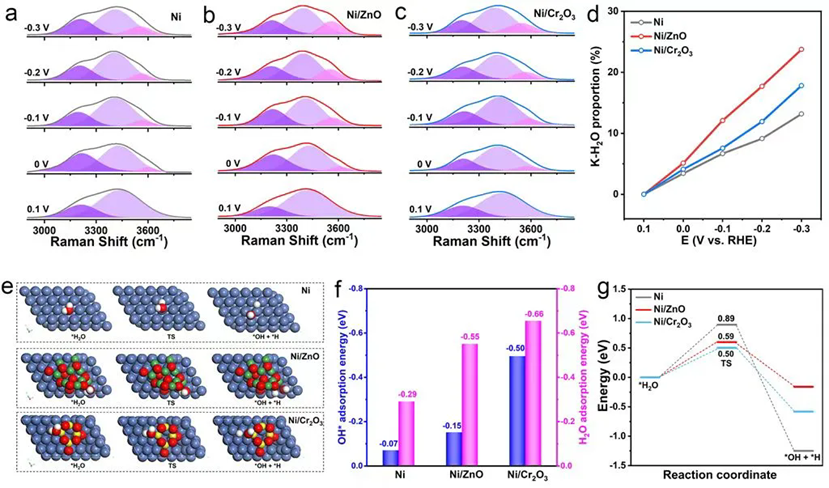
To further validate the enhanced HER kinetics of the Ni/ZnO catalyst resulting from its optimal *OH binding capability, mechanistic study was conducted through in situ spectroscopic characterization and DFT calculations. In situ Raman spectroscopy showed that Ni/ZnO exhibited a higher proportion of K·H2O, indicating it induced a more ordered interfacial water structure. DFT calculations demonstrated that due to its moderate *OH adsorption capability, Ni/ZnO not only promoted the water dissociation process but also avoided the difficult desorption caused by excessively strong *OH adsorption (e.g., as observed in the Ni/Cr2O3 catalyst). These results are consistent with the volcano plot trend, confirming that the moderate *OH binding capability of Ni/ZnO balances water dissociation and *OH desorption, achieving optimal HER activity.
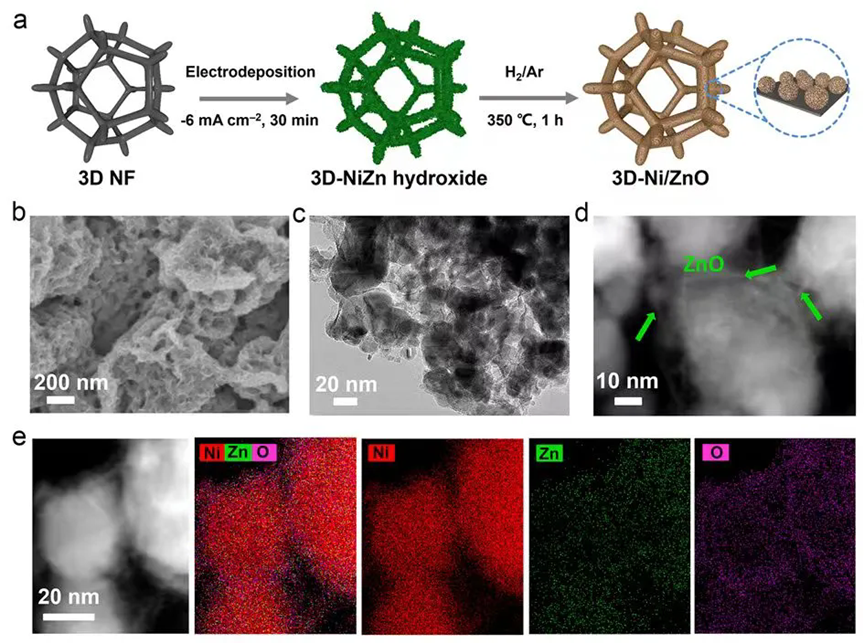
Based on the optimal active composition of Ni/ZnO, a heterostructure catalyst (3D-Ni/ZnO) was further constructed on a three-dimensional nickel foam substrate via an in situ electrodeposition method to expose more active sites and enhance HER performance. Electron microscopy characterization revealed that Ni/ZnO was uniformly loaded onto the nickel foam skeleton, with Ni nanoparticles encapsulated by ultrathin ZnO nanosheets. Additionally, this preparation method is suitable to fabricate large-area catalyst electrodes.
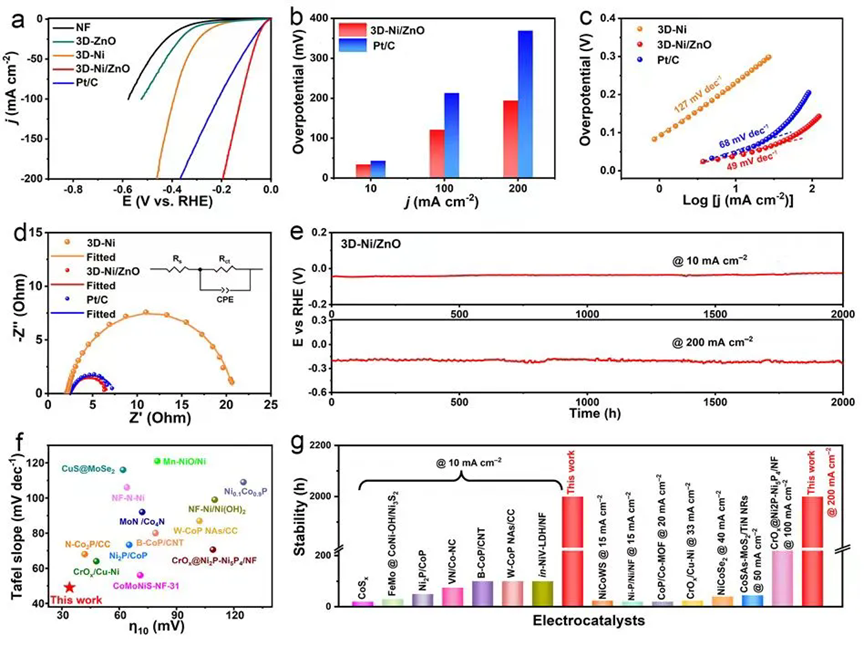
HER performance tests showed that the 3D-Ni/ZnO catalyst exhibited excellent HER activity, requiring overpotentials of only 34, 121, and 194 mV to achieve current densities of 10, 100, and 200 mA cm⁻², respectively, significantly surpassing the Pt/C catalyst. Tafel slope analysis indicated that the HER mechanism for 3D-Ni/ZnO shifted from the Volmer-controlled step observed for the Ni catalyst to the more efficient Volmer-Heyrovsky mechanism. Furthermore, 3D-Ni/ZnO demonstrated outstanding durability, operating stably for over 2000 h at current densities of 10 and 200 mA cm⁻2. Overall, the developed 3D-Ni/ZnO catalyst exhibits superior activity and stability compared to most neutral HER catalysts reported in the literature.